carboxylate ir stretch
The treatment can be applied to the eyelids neck face arms. Sutton Gabriel da Silva and George V.
The C O Bond Part Iii Carboxylic Acids
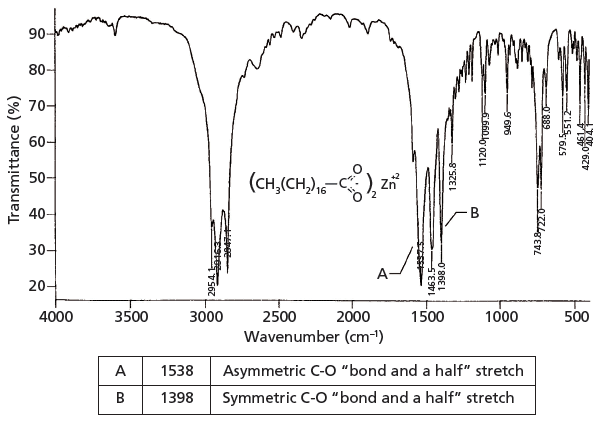
A CO stretch falls at about 1700 1 and a C-O stretch typically falls at about 1200 5.

. And to a lesser extent in differentiating between different kinds of C-H stretches and of course OH and NH. The infrared spectra of six aqueous carboxylate anions have been calculated at the m05-2xcc-pvtz level of theory with the smd solvent model and validated against experimental data from the literature over the region of 1700 cm -1 to 1250 cm -1. One common use is to identify the environment of carbonyl groups CO.
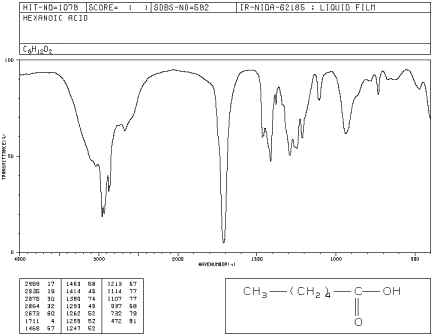
1780 - 1710 s 1690 - 1630 s The carbonyl stretching absorption is one of the strongest IR absorptions and is very useful in structure determination as one can determine both the number of carbonyl groups assuming peaks do not overlap but also an estimation of which types. Carboxylic acids easily dissociate into a carboxylate anion. It is reported that the first IR spectroscopic observation of carboxylate stretching modes in free space ie in the complete absence of solvent or counterions is reported.
The absence of CO HCO H 2 CO and H 3 CO in the in situ IR and XPS spectra is probably a consequence of the low residence time of these species on the surface under reaction. The carbonyl stretch CO of saturated aliphatic aldehydes appears from 1740-1720 cm -1. Why is the carbonyl IR stretch in an ester higher than in a ketone.
The average of 1700 and 1200 is 1450 close to where the carboxylate stretching peaks fall. Correlation between bonding geometry and stretching mode wavenumber shifts Catherine. The carbonyl group of an ester therefore has a C-O double-bond character than does the carbonyl group of a ketone so the former is stronger and harder to stretch.
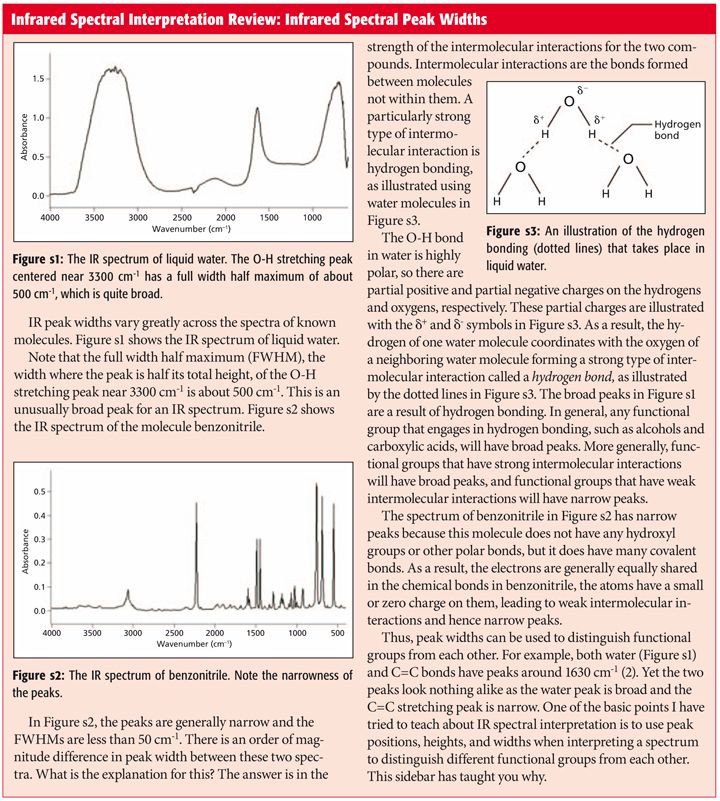
IR carboxylate stretching modes are widely used to infer if the geometry of the bonding is monodentate or bidentate. The carbonyl stretch CO of a carboxylic acid appears as an intense band from 1760-1690 cm -1. Gas-phase spectra of a series of benzoate anions have been recorded and compared to condensed-phase spectra revealing the profound influence of the environment on the symmetric and antisymmetric carboxylate stretch modes.
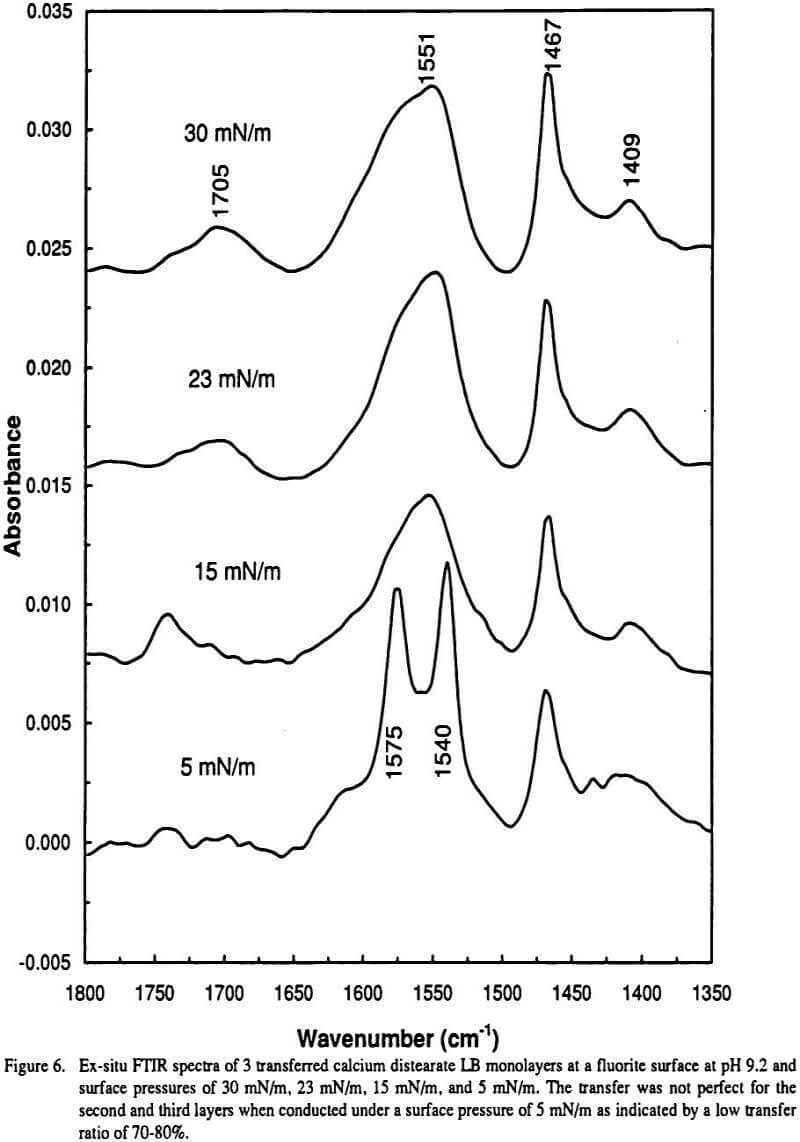
Gas-phase spectra of a series of benzoate anions have. Modelling the IR spectra of aqueous metal carboxylate complexes. Theoretically then a C-O bond and a half stretch might fall at the average peak position of these two carbonoxygen stretching vibrations.
Abstract A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal center can be used to infer if the geometry of the bonding is monodentate or bidentate. Such acids typically have pK a of less than 5 meaning that they can be deprotonated by many bases such as sodium hydroxide or sodium bicarbonate. Share Improve this answer answered May 28 2019 at 259 Rawan 1 1.
Like the CC bond the CC bond stretch is not a very reliable functional group. We know that the wave number is dependent on two things from an earlier video. We report the first IR spectroscopic observation of carboxylate stretching.
With respect to metalcarboxylate interactions the most useful characteristic bands obtained by FTIR are a strong asymmetric COO stretching vibration ie νasym COO and a somewhat weaker symmetric COO stretching vibration ie νsym COO. Carboxylate ions can be formed by deprotonation of carboxylic acids. Carboxylate anions exhibit characteristic vibrational spectra in the infrared IR which typically includes an intense peak due to symmetric stretching of the COO functional group as well as a peak due to antisymmetric stretching.
Carboxytherapy is a treatment for cellulite stretch marks and dark under-eye circles. Lets compare the strength of that bond to a carbon-hydrogen bond where the carbon is SP3 hybridized. This is the bond stretch for nitrogen-hydrogen so thats this bond stretching right there.
You should bear in mind however that the concentration limits of detection by IR-spectroscopy is about 1. So in magenta this is the nitrogen-hydrogen bond stretch. A widely used principle is that shifts in the wavenumber of carboxylate stretching modes upon bonding with a metal centre can.
We report the first IR spectroscopic observation of carboxylate stretching modes in free space ie in the complete absence of solvent or counterions. Such as intensity of the IR-band at 1638 cm -1. The exact position of this broad band depends on whether the carboxylic acid is saturated or unsaturated dimerized or has internal hydrogen bonding.
RCOOH NaOH RCOONa H 2 O Resonance stabilization of the carboxylate ion. As in ketones if the carbons adjacent to the aldehyde group are unsaturated this vibration is shifted to lower wavenumbers 1710-1685 cm -1. We report the first IR spectroscopic observation of carboxylate stretching modes in free space ie in the complete absence of solvent or counterions.
3700 - 3500 m. University of Central Missouri. The frequency of the carboxylate stretching vibration near 1550 cm.
This region corresponds to the stretching modes of the carboxylate group and is often. We have tested this principle with ab initio modeling for aqueous metal carboxylate complexes and have shown that it does indeed hold. 1 in the dianion of 4-hydroxybenzoic acid to 1650 cm.
Bond in a carboxylic acid has a partial double-bond character that is due to resonance electron donation. A linear standard curve using anhydrous sodium salicylate has been obtained from the plot of carboxylate COO concentration vs. Stretch-Induced Crystallization of Cellulose Spun from Ionic Liquid Solution.
It originated in French spas in the 1930s. The weight of the IR band area using the COO antisymmetric stretch vibration at 1580 cm 1. These tend to come in the form of aldehydes and ketones esters amides.
The C O Bond Part Iii Carboxylic Acids
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts

Lec15 Ir Spectra Of Carboxylic Acids Amides And Esters Youtube
The C O Bond Part Iii Carboxylic Acids

How Range Peak Ir Spectrum For Carboxylic Acid Salts Ex Calcium Lactate

Infrared Spectrum Of Ethanoic Acid Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Ethanoic Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes
Ir Infrared Absorption Bands Of Carboxylate
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts
The Carbonyl Group Part V Carboxylates Coming Clean
22 2 Spectroscopy Of Carboxylic Acid Derivatives Chemistry Libretexts
21 3 Spectroscopy Of Carboxylic Acids Chemistry Libretexts

How Range Peak Ir Spectrum For Carboxylic Acid Salts Ex Calcium Lactate

Interpretation Of Carboxylic Acid Ir Spectra Youtube

Infrared Spectrum Of Benzoic Acid C7h6o2 C6h5cooh Prominent Wavenumbers Cm 1 Detecting Functional Groups Present Finger Print For Identification Of Benzoic Acid Image Diagram Doc Brown S Advanced Organic Chemistry Revision Notes


Comments
Post a Comment